Science
The Science Behind OneTest™ for Cancer
OneTest™ is a multi-cancer early detection blood test (MCED) that predicts an individual’s risk of being identified as having cancer in the coming 12-month period.
What is an MCED (Multi-Cancer Early Detection) test?
An MCED is a blood test that detects and analyzes the levels of multiple biomarkers (DNA or protein) that signal the presence of many types of cancer. The goal of an MCED is to detect a cancer early, before symptoms appear as earlier intervention often results in better outcomes.
In 2022 MCEDs became a key component of President Biden’s “Cancer Moonshot” program. As a result, the U.S. National Cancer Institute plans to support large population studies of MCEDs beginning in 2024. (https://prevention.cancer.gov/major-programs/mcd).
How is OneTest™ different from other MCEDs?
MCEDs are a relatively new type of cancer screening approach that began to enter the market in 2019 and 2020. Most MCEDs measure circulating tumor DNA (ctDNA). 20/20’s OneTest for Cancer, in contrast, measures tumor antigens, proteins that tumor cells release in the bloodstream or present on their surface that are recognized by the immune system
These protein biomarkers have FDA-approved in-vitro diagnostic (IVD) test kits available through major US diagnostic manufacturers. The use of FDA-approved tests, run in a CLIA certified laboratory, ensures the analytical performance of these tests. Furthermore, by using these off-the-shelf individual tests, the costs of OneTest™ can be kept to a minimum making it one of the most affordable MCEDs on the market.
Unlike most other available MCEDs, OneTest™ was developed in an entirely asymptomatic, real-world cohort of individuals, meaning that at the time of testing, patients were not known to have cancer and all identified cancers were detected in the post-test period. Most developers use known cases of disease for test development which allows them to use smaller study cohorts. This latter approach may result in biomarker signatures that have greater ability to differentiate between cancer and non-cancer (greater sensitivity and specificity), but less overall utility, as they do not detect cancer as early.
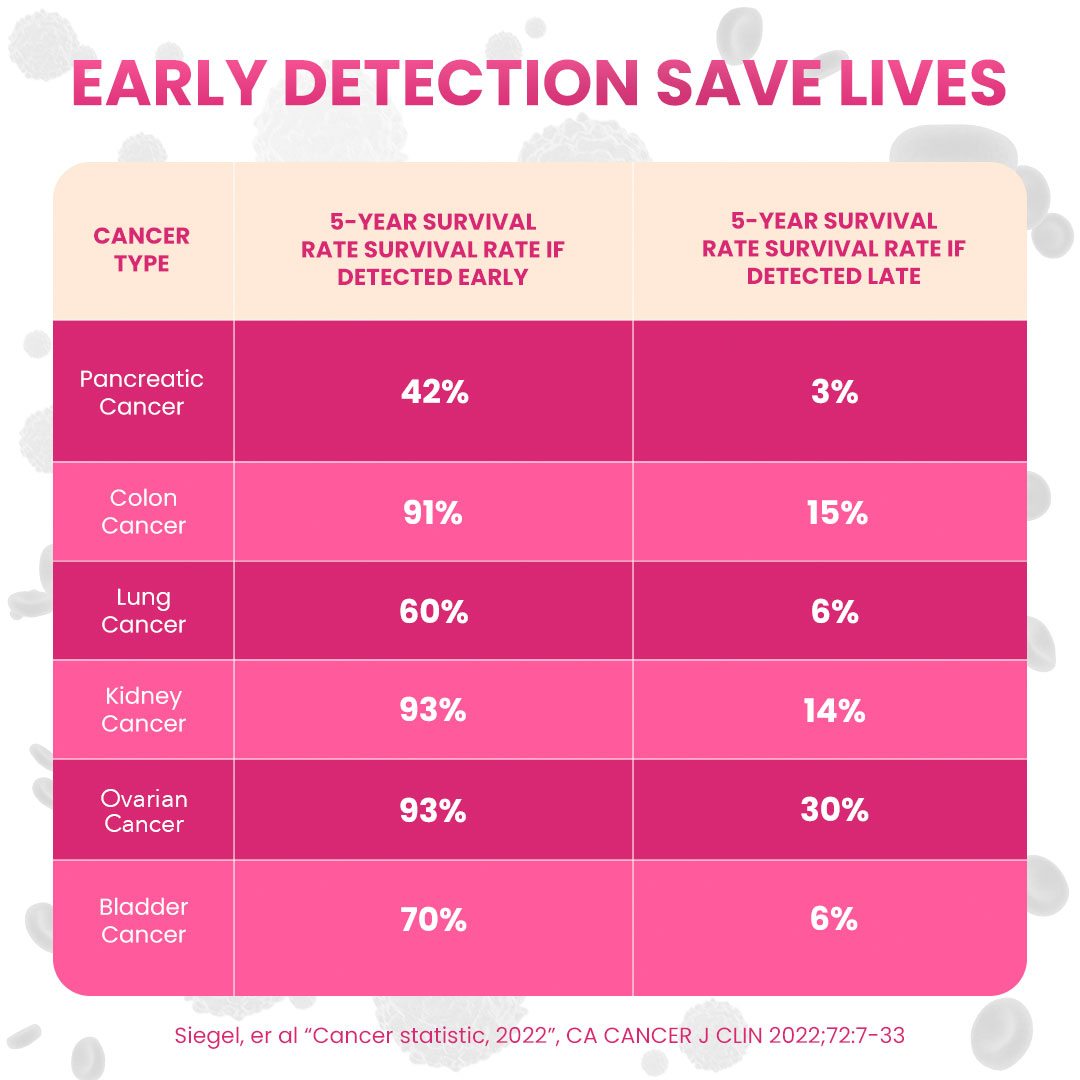
About OneTest™
What are the markers that are used in OneTest™?
OneTest™ measures a panel of tumor markers including AFP, CEA, CA19-9, CYFRA21-1, and PSA for males and AFP, CEA, CA19-9, CYFRA21-1, CA125, and CA15-3 for females. These biomarkers are well known and established in the oncology field, yet with the exception of PSA for prostate cancer, are generally considered insufficient for cancer diagnosis on their own and are primarily used only for monitoring effectivity of cancer therapy and/or for detecting disease recurrence. Each of the markers are known to be elevated in specific cancer types, yet their levels are not always consistent and may vary from patient to patient. The cancers associated with these markers include over 20 of the most commonly occurring cancers (e.g. lung, liver, colon, prostate, breast, etc.).
- Alpha-fetoprotein (AFP) – AFP is an albumin-like glycoprotein that is normally associated with fetal development. It is generally elevated during pregnancy, liver regeneration, alcohol-mediated cirrhosis and hepatitis. As a tumor marker it is associated with liver cancer (HCC), germ cell cancers and other GI related cancers. AFP is currently used to monitor treatment of liver cancer.
2. Carcino-embryonic antigen (CEA) – CEA is a protein involved in cell adhesion that is primarily expressed during fetal development. It can be elevated in a number of benign conditions including smoking, cirrhosis of the liver, jaundice, diabetes, pancreatitis, chronic renal failure, colitis, diverticulitis, irritable bowel syndrome, pleurisy and pneumonia. As a tumor marker it is especially associated with colorectal cancers, other GI cancers and lung cancer.
3. Carbohydrate (cancer) antigen 19-9 (CA19-9) – CA19-9 is also an oncofetal glycoprotein with low level expression in the adult pancreas, biliary duct, stomach, colon and endometrial and salivary epithelia. It is a sialylated Lewis a antigen. CA19-9 is elevated in pancreatic and other GI-related cancers.
4. Cytokeratin fragment 21-1 (CYFRA21-1) – Cytokeratins are structural proteins forming the subunits of epithelial intermediate filaments. Fragments of these proteins may be detected in serum and elevated levels of CYFRA 21-1 are associated with lung cancers.
5. Prostate Specific Antigen (PSA)5,6 – PSA is a glycoprotein that has a close structural relationship to the glandular kallikreins and functions as a serine proteinase. Elevated serum levels of PSA are indicative of prostate pathology but can be due to both benign (benign prostatic hyperplasia (BPH)) and/or malignant disease (prostate cancer). PSA is currently used as a diagnostic for prostate cancer.
6. Carbohydrate (cancer) antigen 125 (CA125) – CA125 is a glycoprotein whose levels in serum are most associated with ovarian cancers. CA125 is also elevated in other female cancers and in GI tumors. It may also be elevated in benign conditions including during specific phases of the menstrual cycle, pregnancy, cirrhosis, hepatitis, endometriosis, ovarian cysts and pelvic inflammatory disease. CA125 has been used as part of several diagnostic panels for diagnosis of ovarian cancer.
7. Carbohydrate (cancer) antigen 15-3 (CA15-3) – CA15-3 is another glycoprotein, the presence of which in serum is associated with breast cancer. Currently CA15-3 is only used postoperatively to monitor of breast cancer recurrence.
How was OneTest™ developed?
OneTest™ was developed using data from 27,938 asymptomatic individuals: incorporating age, gender and levels of multiple tumor biomarkers. The development began with an exploration of tumor marker values by our collaborators at Chang Gung Medical Hospital (CGMH) in Taiwan in a large cohort of real-world, asymptomatic individuals whose lab values were measured during routine health check-up visits over a 12-year period. Simple analysis of a panel of eight tumor biomarkers in this patient cohort demonstrated high sensitivities for the detection of liver cancer (91%), lung cancer (75%), prostate cancer (100%), and colorectal cancer (77%) in this asymptomatic, pre-diagnostic population.
In subsequent work our collaborators proceeded to apply machine learning (ML) / artificial intelligence (AI) methods to improving the analysis of the data. Resulting in the development of gender specific algorithms to predict overall risk of multiple different cancer types in asymptomatic individuals. These algorithms demonstrated even higher levels of sensitivity and specificity.
Direct collaborative work between scientists at both 20/20 GeneSystems and Chang Gung Medical Hospital led to further improvements in the algorithms by incorporating clinical factors (age) into the applied mathematical models. A further analysis of the correlation between risk scores and time to diagnosis in the patient cohort allows for a more complete understanding of appropriate follow-up for moderately vs. substantially higher risk scores.
Scientists at 20/20 GeneSystems have expanded the existing data supporting OneTest™, by incorporating data from over 160,000 individuals in a major hospital system in China. We have also continued to apply novel AI approaches to analyze the data and explore the benefits of longitudinal biomarker data.
How does AI/ML improve test results?
Artificial Intelligence (AI) and Machine Learning (ML) are mathematical and computer-based methods for analyzing large datasets and integrating multiple data fields. Well standard methods use simple cutoff values for individual biomarkers, AI/ML methods are far more sophisticated and can employ a sliding scale for each biomarker dependent on other biomarker values. By way of example, different thresholds for one marker can be applied dependent on both subject age and value of a second biomarker. These approaches can greatly increase test sensitivity and specificity by comparing each subject to the individuals in the training/validation cohort who are most similar in both biomarker values and clinical factors.
What are the performance characteristics of OneTest™?
Performance of diagnostic tests is most often characterized by test “Sensitivity” and “Specificity”. Sensitivity is the percentage of cancers correctly classified by the test as cancers (true positive rate). Specificity is the percentage of non-cancers correctly classified by the test as non-cancers (false positive rate). The higher the test sensitivity, the fewer the number of missed cancer cases. The higher the test specificity, the fewer the number of false positives cases. In general, there is a trade-off between sensitivity and specificity, meaning that if a threshold is established to identify 100% of cases of disease, it will likely identify a large number of false positives, while if the threshold is set to minimize false positive it will also miss a number of disease cases. Holding specificity at ~80%, the sensitivity of OneTest™ for any cancer in males is ~82% and in females is ~62%. This is a significant improvement over a simple threshold model of looking for any one tumor marker to be elevated above its test manufacturer threshold value. While these levels of sensitivity and specificity might be considered relatively modest for a direct diagnostic test, scientists at 20/20 GeneSystems believe that they are appropriate for a risk predictor test like OneTest™. This is especially true given that the most likely follow-up to an elevated risk score will be follow on testing with the same or other MCED tests.
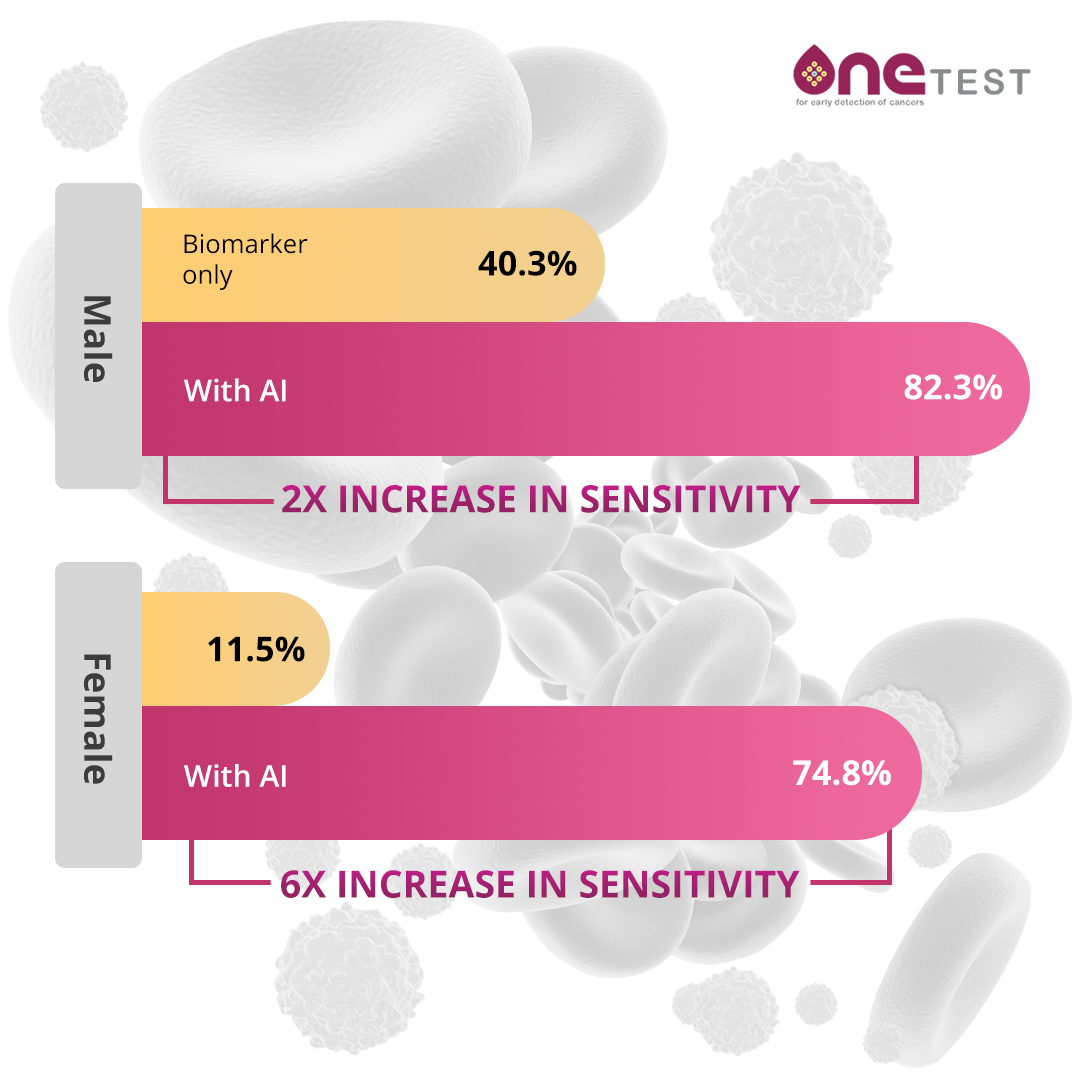
How should OneTest results be utilized?
OneTest™ does NOT replace other early detection tests such as colonoscopy, mammogram, low dose computed tomography, etc. These tests should continue to be utilized per standard medical guidance. OneTest™ is intended as an adjunct to these and other cancer screening modalities.
The OneTest™ report will indicate both the resulting values of the individual biomarker tests and a composite OneTest™ score. The individual biomarker values are reported together with the test manufacturer supplied reference range which in most cases has been determined for monitoring of already diagnosed cancers and not specifically for early detection. Elevation of these values may be indicative of an ensuing cancer. The OneTest™ score is based on our proprietary algorithm which considers more subtle changes in biomarker values and combinations of these values in order to detect cancer earlier. The OneTest™ score is reported as a risk score; the value indicates the percentage of individuals with biomarker values like yours, at your age and considering your gender, who were identified as having cancer within a year of testing. In simple terms, a OneTest™ score of 5 indicates that 5% of individuals with biomarker values like yours were identified as having cancer within one year of the test. OneTest™ scores range from 1 to 30. Values of 1 indicate no increased risk over the general population.
An elevated OneTest™ score is only a risk predictor and NOT diagnostic for cancer. The response to an elevated OneTest™ score should be determined by a health care professional who is familiar with the individual patient’s health history. In most instances follow-up will include further screening or diagnostic testing which may include repeat testing with OneTest™ at a greater frequency, the use of another MCED, or cancer-type specific diagnostic tests. OneTest™ is NOT meant for symptomatic individuals or for monitoring for disease recurrence.
Contact us
DETECT CANCER EARLY, WHEN TREATMENT WORKS BEST
OneTest provides the peace of mind that comes from knowing you’re taking proactive steps to protect your health.